
What we do
Genostics is an Australian medical, education and consultancy company. Based in Sydney, we collaborate with various international laboratories to bring the very best in personalised cancer testing to the Australian and New Zealand medical communities.
Science has brought us into the age of ‘Personalised Medicine’ and Genostics is pioneering the provision of advances in Molecular Medicine to clinical practice, thus assisting health practitioners in optimising treatment outcomes and patient care.
We engage with pioneering practitioners who seek to discover the exact nature of each patient’s cancer. These practitioners use the tests that Genostics facilitates to help design personalised treatment plans and to closely monitor the effectiveness of therapies.
Specialising in the field of Oncology, we are proud to be bringing the future of personalised cancer testing to patients, today.
Genostic tests are not diagnostic tests, but can be used in addition to standard protocols as a tool for understanding the individual nature of a person’s cancer. They can be used to as a tool in the determination of prognostic factors and to monitor the effectiveness of treatment. These tests do not replace current screening, testing or clinical practice protocols.
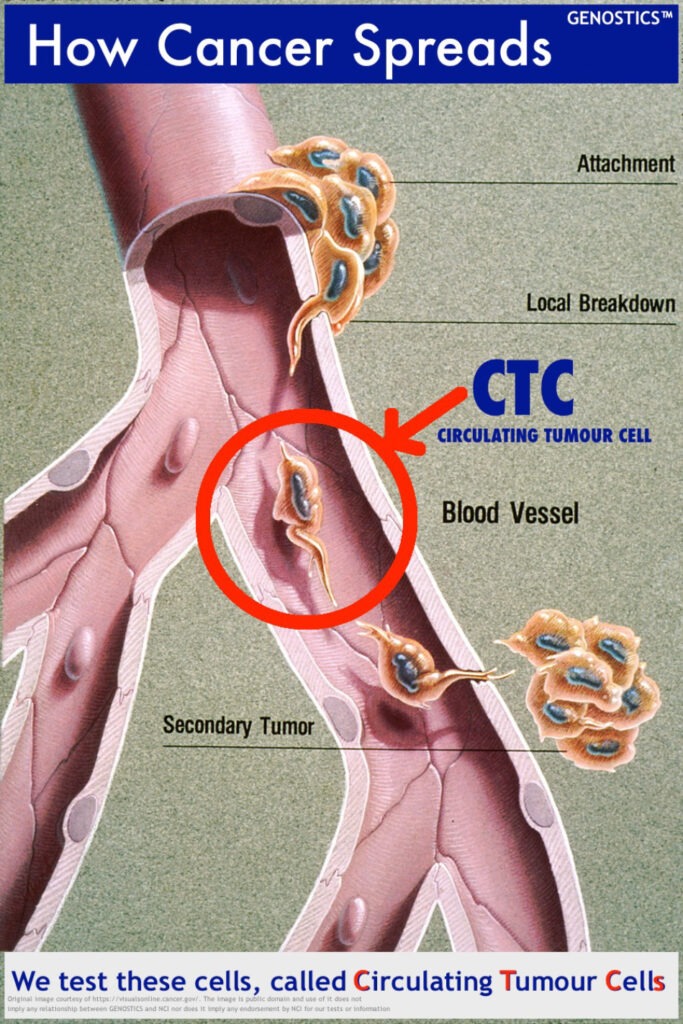
CIRCULATING TUMOUR COUNT (CTCs)
Escapee cancer cells in the blood, or ‘CTCs’ as they are known, can be found by a simple blood test. A CTC Count test finds existing escapee cancer cells (CTCs) in a blood sample and counts them. Monitoring changes in cancer activity over time is made easy with this test…
The cost of the Maintrac CTC testing including the shipping to Germany is $845.00 AUD

What is circulating tumor DNA and how is it used to manage cancer?
Circulating tumor DNA (ctDNA) is found in the bloodstream and refers to DNA that comes from cancerous cells and tumors. Most DNA is inside a cell’s nucleus. As a tumor grows, cells die and are replaced by new ones. The dead cells get broken down and their contents, including DNA, are released into the bloodstream. ctDNA are small pieces of DNA, usually comprising fewer than 200 building blocks (nucleotides) in length.
The quantity of ctDNA varies among individuals and depends on the type of tumor, its location, and for cancerous tumors, the cancer stage.
Detection of ctDNA can be helpful in the following cases:
- Detecting and diagnosing a tumor. Because tumor DNA has acquired multiple genetic changes (variants), leading to tumor development, ctDNA is not an exact match to the individual’s DNA. Finding DNA with genetic differences aids in tumor detection. Diagnosing the type of tumor using ctDNA can reduce the need for getting a sample of the tumor tissue (tumor biopsy), which can be challenging when a tumor is difficult to access, such as a tumor in the brain or lung.
- Guiding tumor-specific treatment. Analyzing the genome of tumor cells using ctDNA can help doctors determine which treatment will be most effective. Currently, however, approval from the U.S. Food and Drug Administration for ctDNA testing to personalize cancer treatment is limited.
- Monitoring treatment. A decrease in the quantity of ctDNA suggests the tumor is shrinking and treatment is successful.
- Monitoring periods with no symptoms (remission of cancer). A lack of ctDNA in the bloodstream indicates that the cancer has not returned.
